Increased explosive power from metal nanocomposites
Why are metal nanocomposites interesting?
Producing high-energy explosives with increased energy is impractical due to increased sensitivity. Metal composite powders prepared with reactive metals (magnesium, aluminum, silicon, boron) as fuels and ceramic/polymeric oxidizers have superior theoretical energy density as compared to conventional high energy explosives like HMX. The metal fuels also react very exothermically with the decomposition products of explosives such as carbon dioxide and water vapor. To capitalize on these advantages, metallic composites prepared by arrested reactive milling (ARM) are explored as both replacements and additives. Exciting new work shows that when packed cylinders of metal-rich composite powders are shock compressed, very fast non-diffusive mixing of reactants is observable in nano-second timeframe. Reference: S. Matveev, D.D. Dlott, S.K. Valluri, M. Mursalat, E.L. Dreizin, Fast energy release from reactive materials under shock compression, Applied Physics Letters 118, 101902 (2021)
This suggests that the thermodynamic benefits of metallic nanocomposites can be realized with sufficiently fast kinetics.
Can we engineer composites to become more sensitive to shock?
A wide assortment of composites can be prepared by ARM. Any metal or alloy of interest can be chosen as fuel and an oxidizer from a large number of oxides, fluorides, nitrates and polymers can be mixed intimately. Their physical attributes such as particle size distribution, porosity, and degree of separation between fuel and oxidizer can be engineered (Learn more about ARM). By tuning these attributes, the composite’s shock-driven reactivity can be improved. Preliminary work on the effect of porosity shows that porous composite particles develop hotspots when shock compressed.
Current research direction
To find the right composite with favorable composition and physical attributes, a variety of preparations need to be tested. We use our high throughput Shock Compression Microscope to process a large number of samples to find ideal candidates. We explore the behavior of metal composites at three levels of complexity.
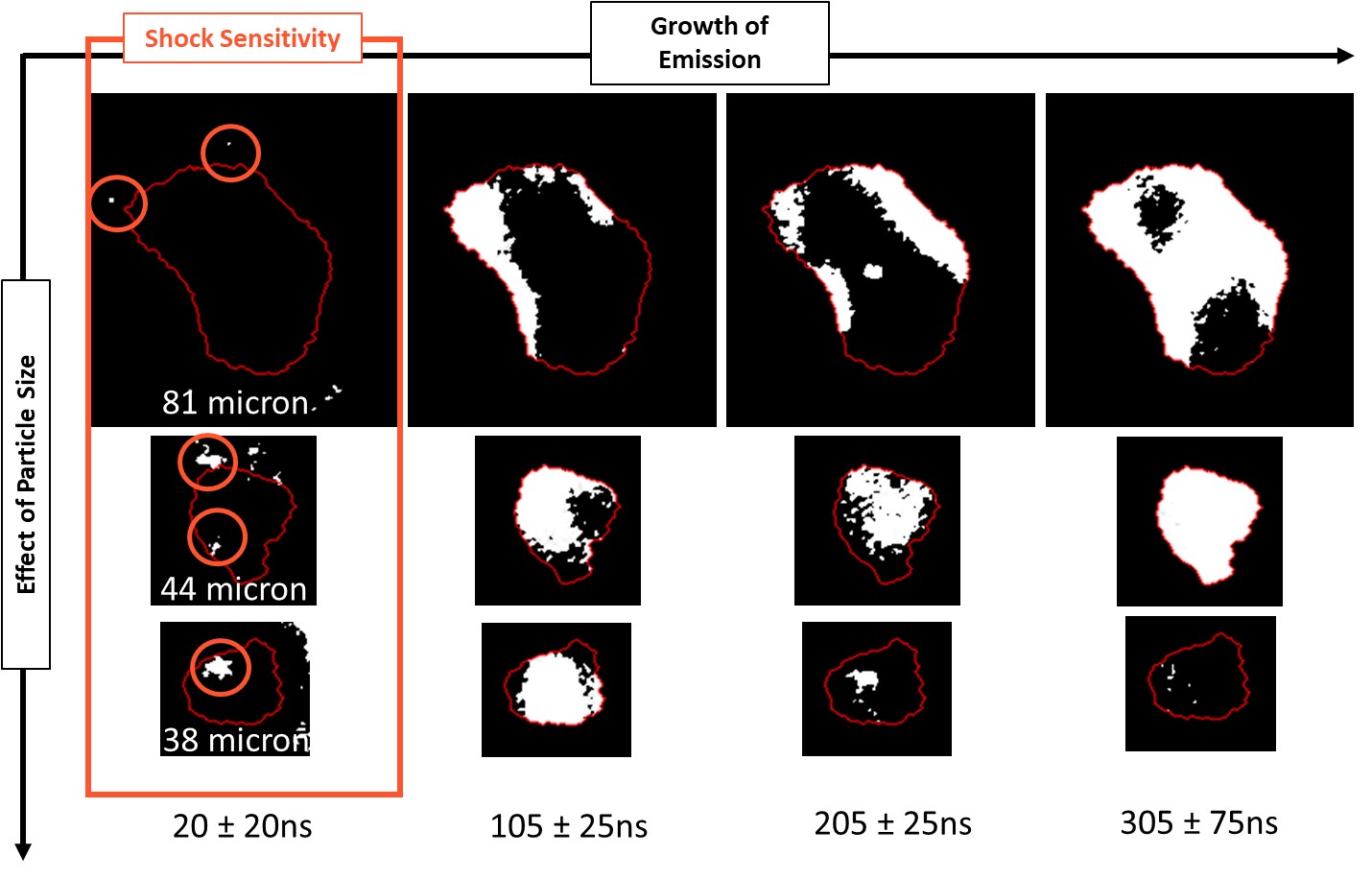
The intrinsic reactivity of particles and their sensitivity to shock without the assistance of packing microstructure is initially explored. Using simultaneous high-speed imaging and pyrometry, we have both spatial and temporal resolution to identify reactivity in micron-sized particles as shown in figures below. Further, role of microstructure where each particle is assisted and/or assists its neighbor, is pursued by using powders that are packed in two and three dimensions. Finally, interesting samples that show fast reactions in previous experiments, are mixed with explosives and their combined behavior is characterized.
The figure below shows the evolution of spatially distributed emission plumes captured by high-speed imaging. In this case, emission within isolated particles is shown. Current efforts are augmented by computer vision-based techniques not only to identify particles but to also identify and track the emission features over time and space.
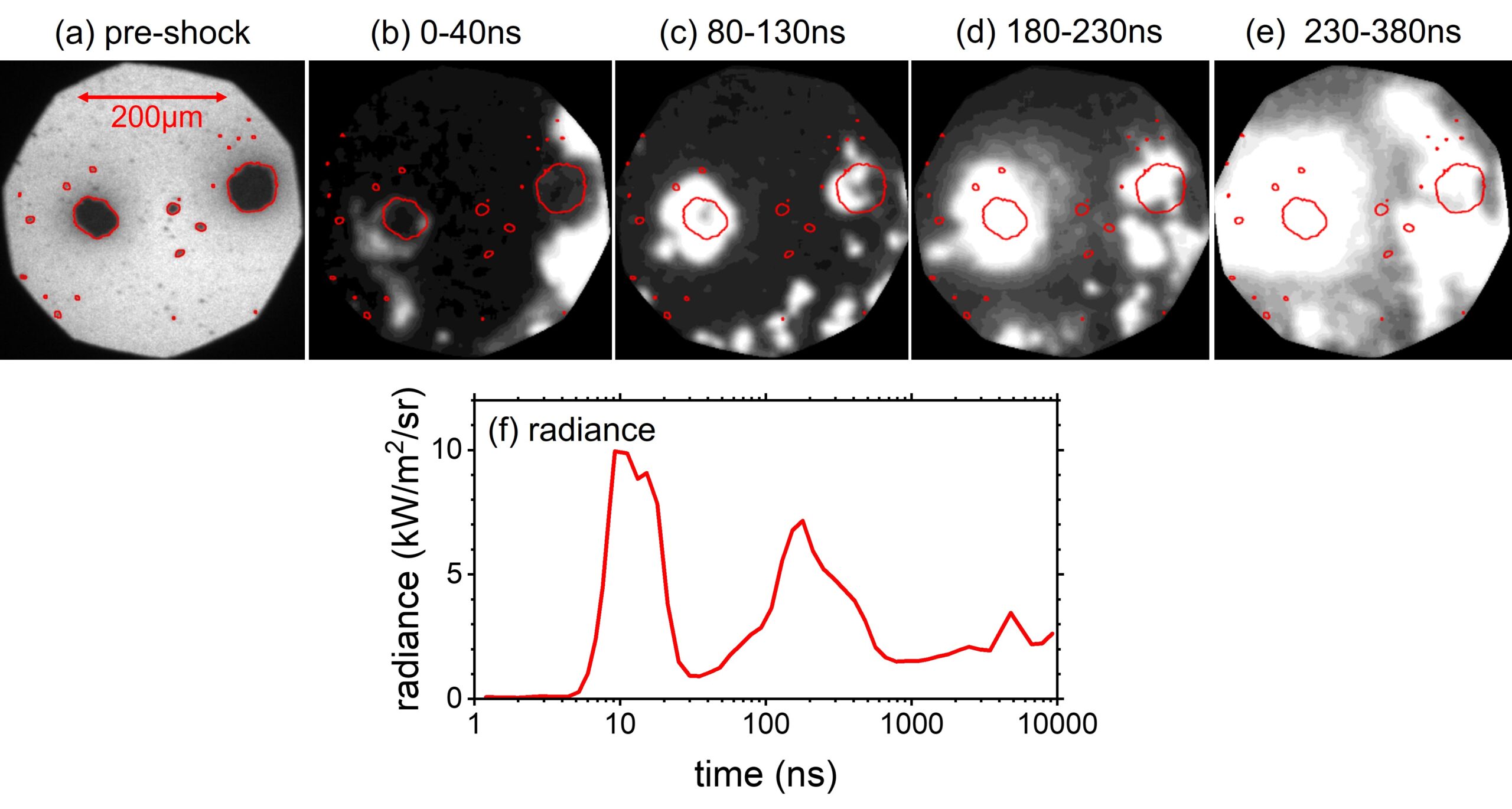
This manner of analysis for the emission identified within particle(s) and/or local region of interest gives us insight to link particle/microstructure features and descriptors not only to hotspot formation and evolution, but also reaction kinetics. Future work shall showcase, temperature distribution over microstructures arranged in a two dimensional plane.
For current details, refer to the ResearchGate project file.